Cut rye (Secale cerealis) or creeping bent grass (Agrostis stolonifera) blades into 8-10 mm lengths and boil them for 10 min. in distilled water. Transfer 2-4 pieces to sterile 10% soil extract in small (35 X 10 mm or 60 X 15 mm) Petri plates.
Inoculate the plates with a small amount of Pythium-colonized agar but transfer as little agar as possible.

A 35 X 10 mm plate can be examined on some traditional compound microscopes, depending upon the working distance between the objective and the stage, thus maintaining the oogonium/antherdium position. Trying to set up a microscope slide and coverslip usually disrupts the position or makes it very difficult to observe.
If an inverted light microscope is available, cultures are more easily examined because the culture is not disrupted and the plate need not be opened.
Record the date of inoculation.
Place the plates in a clear plastic box or on a tray in a plastic bag and incubate them under fluorescent lights at room temperature.
Using a standard or an inverted compound microscope, examine these plates daily to verify that the organisms are Pythium, looking for sporangia, oogonia, antheridia and zoospores.
Record the characteristics using the checklist sheet and attempt a tentative identification based on morphology (van der Plaats-Niterink, A. J. 1981. Monograph of the genus Pythium. Studies in Mycology. Baarn, Centraalbureau voor Schimmelcultures. Monograph No. 21:242 Dick, M. W. 1990. Keys to Pythium. M. W. Dick, Department of Botany, University of Reading, Reading, U.K. 64 pp.).
Refer to the drawings of the characteristics in van der Plaats-Niterink, A. J. 1981. Monograph of the genus Pythium. Studies in Mycology. Baarn, Centraalbureau voor Schimmelcultures. Monograph No. 21:242.
Look for non-septate hyphae (coenocytic), sporangia, sporangia producing zoospores, oogonia, antheridia, and thick-walled oospores. In some species, oospores are very small, not much larger in diameter than hyphae. These are very easy to overlook in culture. Most species are homothallic, but some species are heterothallic and require mating types to obtain oospores. In species with spherical sporangia, sporangia are generally larger than oospores. Sporangia in some species are inflated-filamentous, meaning that they are hyphal-like but have a diameter larger than hyphae. Some species are non-inflated, filamentous meaning that you may see zoospores but not see structures that look any different from hyphae. Look carefully because some species of Pythium form a VERY long tube from the sporangium to the vesicle where zoospores form. The sporangium may be a long distance from the vesicle.
Using Stains in Identifying Pythium Species
Stains can be used to help identify some morphological characters. Moorman does not usually use staining; however some literature suggests it is helpful in identifying characters. Both lactophenol cotton blue and acid fuchsin are used for staining Pythium in culture. As you experiment with staining, it is important to note; the phenol will kill the fungal organism, while the lactic acid preserves its structures (1), therefore do not apply these stains to cultures you wish to continue to observe further growth. Also, any observations you have while staining Pythium, please share this information, as the techniques described below have not been refined or practiced much by our group. Please contact Gary Moorman or Sara May with your observations.
Lactophenol cotton blue stains the chitin present in fungal cell wall tissue (1). To stain Pythium cultures, add 2-4 drops of Lactophenol cotton blue to the petri dish with Pythium inoculated grass blades. Allow it to sit for a couple minutes and observe the stained structures under the microscope.

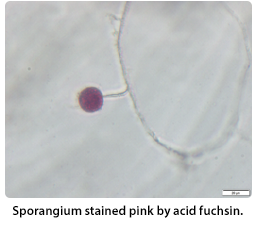
Acid fuchsin stains the reproductive structures pink in culture, assisting with the identification of sporangia and oogonia, and does not stain the hyphae, as observed with lacophenol cotton blue stain. According to Stanghellini et al. (1971), acid fuchsin stains sporangia more intensely than oospores. Simply add 2-4 drops of acid fuchsin to the petri dish with Pythium inoculated grass blades. Allow it to sit for a couple minutes and observe the stained structures under the microscope.
- Sangeetha, J., and Thangadurai, D. 2013. Staining Techniques and Biochemical Methods for the Identification of Fungi. In V. K. Gupta, M. G. Touhy, M. Ayyachamy, K. M. Turner, and A. O'Donovan, Laboratory Protocols in Fungal Biology (pp. 237-257). Springer New York.
- Stanghellini, M. E., and Hancock, J. G. 1971. Sporangium of Pythium ultimum as a survival structure in soil. Thesis--University of California.
The Key
The key is presented as a guide to identifying Pythium to the species level. It is a modification of that created by Anna van der Plaats-Niterink (Van der Plaats-Niterink, A. J. 1981. Monograph of the genus Pythium. Studies in Mycology. Baarn, Centraalbureau voor Schimmelcultures. Monograph No. 21:242). Moorman added some but not all new species of Pythium. In order to add all new species, the key would need to be changed by adding some characteristics and would require extensive examination of cultures of the new species and creation of a new monograph to include DNA sequences. Note that new species are being described fairly frequently and taxonomists are suggesting changes in species and even genus designations. The key is only a guide.
Be flexible in your interpretation of what you observe and observe cultures over time. As you gain experience through looking at many different Pythium cultures, you will begin to be able to differentiate sporangia from oogonia, slightly inflated filamentous sporangia from hyphae, and recognize proliferating sporangia. At first, just reading descriptions is confusing, but the more cultures you observe the process will become much easier.
There are two formats in which the key can be accessed. Use the the links below to access the key:
The key was built using the Lucid Builder program. As shown in the image, when you open the key, the window is divided into four sections: features available, features chosen, entities remaining and entities discarded. The characters from the character list (link to character list in the modules) provided in the modules are present in the Lucid key as "features". Pythium species are present as "entities".
As you look at Pythium cultures, check off the characters you observe on the provided character checklist. Check off characters by expanding the options beneath each main heading of the "features available" section and click on the empty box next to the character you have observed in your culture. As shown in the image, when a character is selected, a check mark will be placed next to that character in the "features available" section. These selected characters will also be copied into the "features chosen" section. In order remove a feature chosen, simply click the checked box next to the unwanted character, and it will become unchecked again. Lastly, as you select the characters you are observing, Pythium species are discarded into the "entities discarded" section, leaving fewer and fewer "entities remaining," or possible Pythium species.
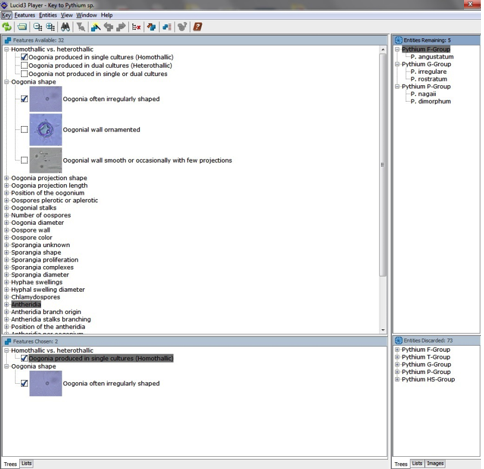
To help you identify Pythium species, images of the characters are available in the Lucid key. To view these images, make sure that "image thumbnails" is selected under the view tab. Simply expand the contents of the "features available" section and click on the thumbnails to enlarge the images. For more information on using Lucid Keys, visit their website.
Be flexible in using the key. If you are not certain of a characteristic, select one option for that characteristic and see where the key takes you. For example, it is often challenging to decide if oospores are plerotic or aplerotic. Simply check one, and see what species remain as you select your other observed characters. When you have eliminated most species, examine the description of the species to which you are led and see if it fits. If the descriptions do not match what you have observed, go back and select a different option for that characteristic and other questionable characters and examine the description of the species to which you come.
Cultures must be observed over time in order to find the various structures noted in the key. It is best to examine a culture frequently and take notes on what is seen because, over time, structures formed soon after starting a culture may be obscured by new growth. AND, for example, what you initially thought were spherical sporangia may turn out to have been young oogonia. Use the check-off list of characteristics to keep track of what is observed.
Some isolates of Pythium do not form oogonia in single or dual cultures. In the key, these are placed into groups with characteristic sporangia or sporangia-like structures:
Group F have filamentous, non-inflated sporangia. Over time as these are observed, you will see what appears to be mycelium but then numerous zoospores will be seen.
Group G have globose, non-proliferating sporangia. A sporangium releases zoospores or germinates to form a hypha but does not give rise to another sporangium.
Group P have globose, proliferating sporangia. A sporangium gives rise to another sporangium within the original sporangium or a hypha forms inside the original sporangium, grows through it, and gives rise to a new sporangium. Or the sporangium releases zoospores.
Group T have toruloid sporangia. Sporangia are more or less cylindrical or finger-like but with knobby swellings.
Group HS have hyphal swellings that look like sporangia but these do not produce zoospores.

