
Watanabe, H., Kageyama, K., Taguchi, Y., Horinouchi, H., and Hyakumachi, M. 2008. Bait method to detect Pythium species that grow at high temperatures in hydroponic solutions. Journal of General Plant Pathology 74 (6):417-424.
Cut creeping bent grass blades (Agrostis stolonifera) into sections. (Just as good in Moorman's experience: rye blades, Secale cereale)

Add sections to a container of water.
Baiting greenhouse irrigation tanks...Place several blades of bent grass between pieces of window screen and suspended this 'trap' in water for 7 days.
Plate blades onto water agar or a selective medium (Part 1: Media for Pythium Culture).
Examine the agar for structures characteristic of Pythium (oogonia, sporangia, appressorium-like structures where the hyphae contact the plastic dish. Look both close to the initial inoculum block AND far from the inoculum block.
Transfer isolates suspected of being Pythium to a selective medium and water agar.
Pythium 'trap' for Large Body of Water
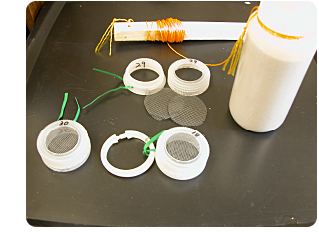
Cut off the threaded section of a plastic jar (29 on left in photo with green twist-tie).
Cut out the center of the lid of the plastic jar (29 on right in photo).
Cut plastic window screen to fit inside the lid.
Sandwich grass blades (not boiled) between two pieces of window screen. Fit that into the lid. Screw the lid onto the threaded section (30 on left in photo).
Use a jar of sand as an anchor. Bait the bottom of the tank by securing a trap to the anchor. Bait the water surface by attaching a trap to a plastic shower curtain ring (18 in photo) and thread the anchor line through the ring so that the floating trap is always on the surface.
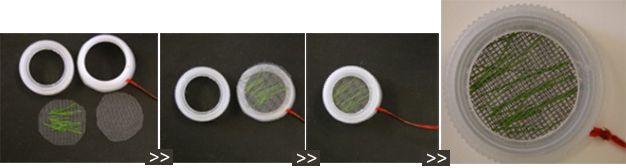
Following incubation, plate the grass blades on a selective medium or water agar.
Verifying that the isolate is a Pythium and for species identification based on morphology

Cut rye (Secale cerealis) or creeping bent grass (Agrostis stolonifera) blades into 8-10 mm lengths and boil them in distilled water for 10 min. Transfer 3-5 pieces to a small Petri plates (35 X 10 mm or 60 X 15 mm; available from VWR or Fisher Scientific) containing sterile 10% soil extract (see preparation of soil extract; Part 2: Media for Pythium Culture ).
A 35 X 10 mm plate can be examined on some traditional compound microscopes, depending upon the working distance between the objective and the stage, thus maintaining the oogonium/antherdium position. Trying to set up a microscope slide and coverslip usually disrupts the position or makes it very difficult to observe.
Refer to the drawings of the characteristics in Van der Plaats-Niterink, A. J. 1981. Monograph of the genus Pythium. Studies in Mycology. Baarn, Centraalbureau voor Schimmelcultures. Monograph No. 21:242.
Look for non-septate hyphae (coenocytic), sporangia, sporangia producing the zoospores, oogonia, antheridia, and thick-walled oospores. In some species, oospores are very small, not much larger in diameter than hyphae. These are very easy to overlook in culture. Most species are homothallic, but some species are heterothallic and require mating types to obtain oospores. In species with spherical sporangia, sporangia are generally larger than oospores. Sporangia in some species are inflated-filamentous, meaning that they are hyphal-like but have a diameter larger than hyphae. Some species are non-inflated, filamentous meaning that you may see zoospores but not see structures that look any different from hyphae. Look carefully because some species of Pythium form a VERY long tube from the sporangium to the vesicle where zoospores form. The sporangium may be a fairly long distance from the vesicle.

